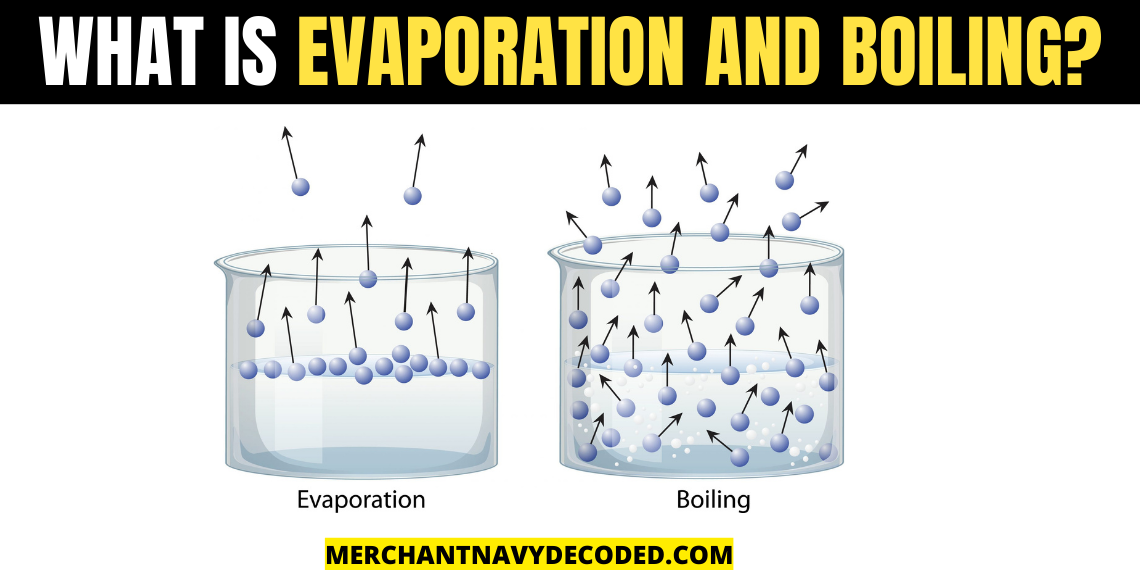
What is Evaporation and boiling?
Evaporation: It is the process in which liquid changes to a vapour state. Evaporation occurs when heat energy forces the bonds which hold the water molecules to break, causing the water to shift from its liquid state to a gaseous state.
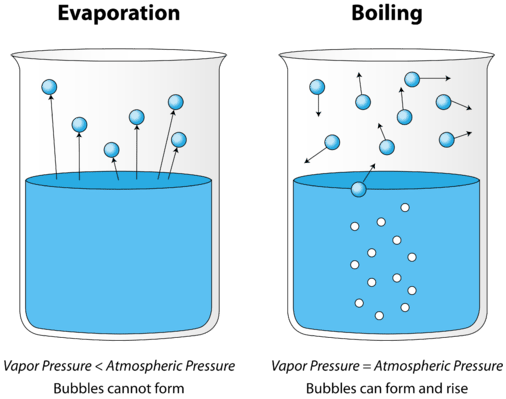
Boiling: Rapid vapourization of liquid when it is heated to its boiling point is known as boiling. The boiling point is the temperature at which liquid changes its state when the vapour pressure is equal to the atmospheric pressure. The boiling point of water is 100 degree Celsius at 1 bar pressure (atmospheric pressure)
Difference between evaporation and boiling
| Evaporation | Boiling |
| 1. Evaporation is a slow process. 2. It takes place on the surface. 3. It can take place at any temperature below boiling point. 4. It cannot form bubbles due to more atmospheric pressure on it | 1. Boiling is a fast process 2. Takes place throughout the volume. 3. At boiling point (in the case of water, it boils at 100 degree Celsius at 1 bar of pressure). 4. It forms bubbles and rises due to the increase of vapour pressure than atmospheric pressure |
What is Vapour pressure?
Vapour pressure: It is the pressure exerted by vapours of liquid on the surface of the liquid when equilibrium is established between liquid and its vapour is known as the vapour pressure of the liquid.

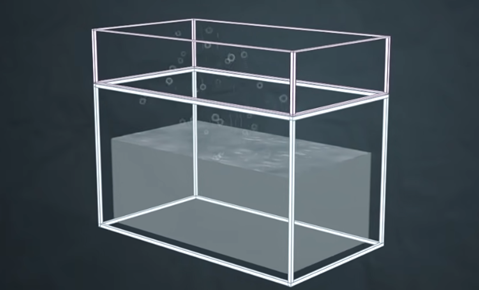
In fig (a) we can see that in the open container, evaporation is taking place, and water vapours are going out of the container. In fig (b) there is a lid placed upon the container due to which water vapours are not able to go out of the container, the water vapours hit the lid and turn back to the liquid and condensation takes place. When the rate of evaporation is equal to the rate of condensation happening it is said to be in equilibrium and this is known as vapour pressure.
What is the role of pressure on boiling?
Role of pressure on boiling: The role of pressure on boiling is that as the pressure on a liquid increases, its boiling point also increases, and as the pressure decreases, its boiling point decreases. This is because increasing pressure on the surface of the liquid raises the vapour pressure required for the liquid to boil. Conversely, decreasing the pressure lowers the vapour pressure required for boiling, and hence, the boiling point decreases.
For example, at sea level, water boils at 100 degrees Celsius at 1 bar pressure, but at high altitudes where the atmospheric pressure is lower, the boiling point of water is lower as well. In areas with higher atmospheric pressure, such as deep-sea environments, the boiling point of water is higher than at sea level. In summary, the boiling point of a liquid is dependent on the pressure surrounding it, with increasing pressure raising the boiling point and decreasing pressure lowering the boiling point.
Where will water boil faster, on mountains (Mussoorie) or on sea level (Goa)?
Water boils faster at lower atmospheric pressures, which are typically found at higher altitudes, such as in mountainous regions. This is because the boiling point of water decreases as atmospheric pressure decreases.
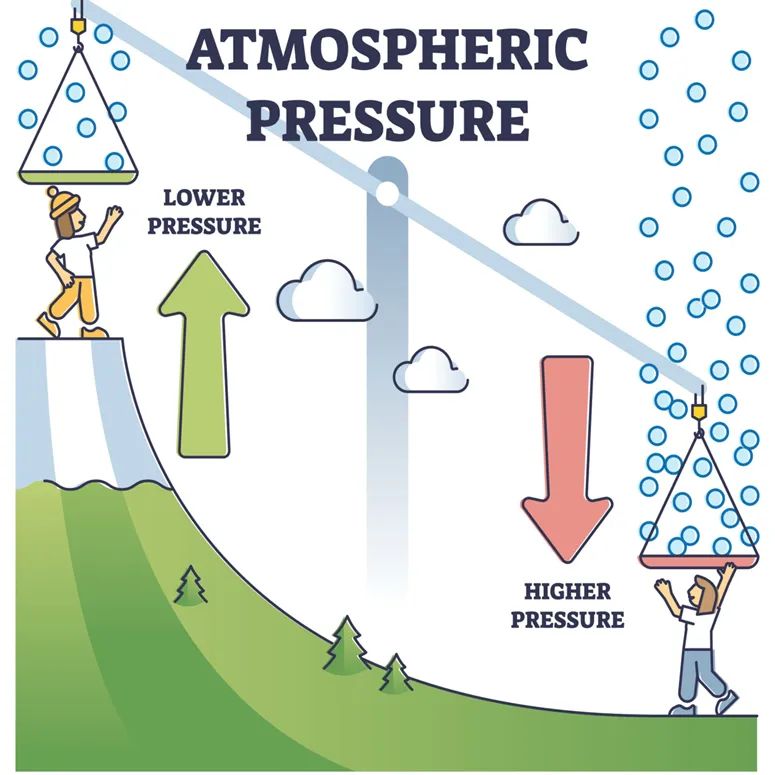
At high altitudes, such as in mountainous regions, the atmospheric pressure is lower compared to sea level, and therefore, water will boil at a lower temperature than it would at sea level. This means that water will boil faster in mountains than at sea level.
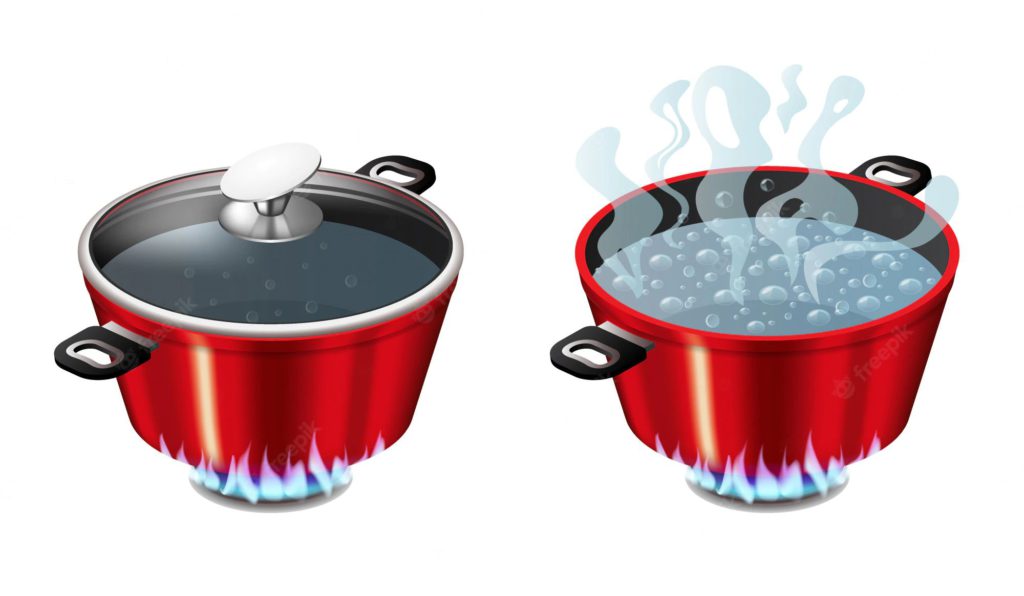
Why does food cook faster in a pressure cooker?
If we take a pressure cooker and a pot. We can see from the above figure that when we give heat energy and boil water on both items we will see that water is boiling at a much faster rate in the pressure cooker than in the pot. It is due to the effect that the pressure cooker is a sealed environment due to the lid upon it which does not allow the vapour molecules of water to move out. In the pressure cooker, the pressure will increase till the time when it will be able to lift the pressure releasing safety valve, increasing the pressure will increase the boiling point of the water.
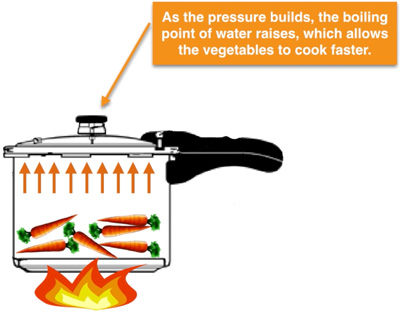
On the other hand, in the frying pan, there is no lid on it and that’s why the atmosphere is acting upon it which is 1 bar pressure less than the pressure in the pressure cooker.
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.


[…] In a smoke tube boiler the exhaust gas or flue gas flows inside the tube from the combustion chamber and heats up the surrounding water and turns it into steam. […]
[…] which is a closed vessel where they heat the water and convert the water into […]