Distillation
Distillation is a separation process that involves heating a liquid mixture to creating vapour and subsequently the vapour is condensed back to liquid form.
The distillation process involves heating a mixture of two or more liquids in a vessel, causing them to evaporate. As the vapour rises, it enters a condenser where it is cooled and returned to its liquid form. The condensed liquid is then collected in a separate container. This process is based on the fact that different substances have different boiling points, and therefore they will vaporize and condense at different temperatures.
Uses: Separating salt from seawater
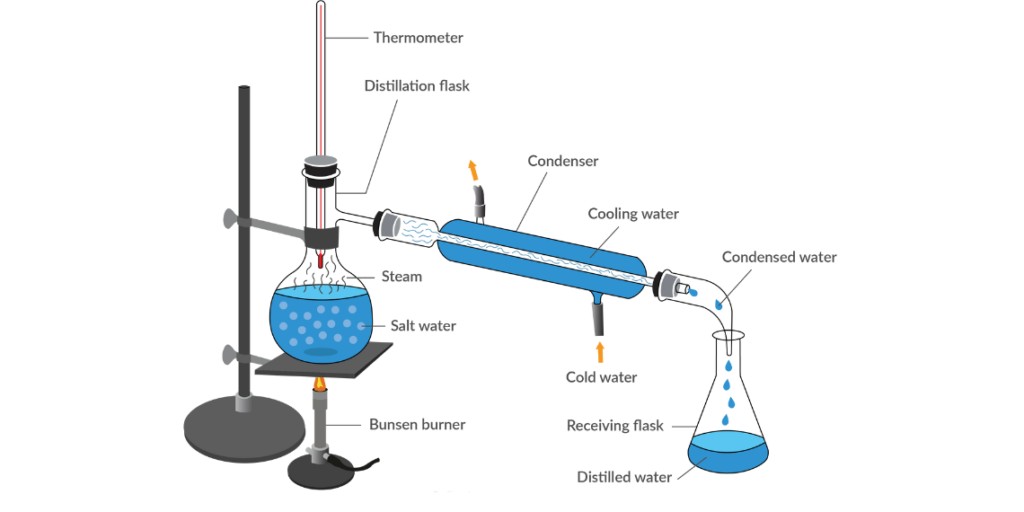
The diagram depicts a simplified distillation process for separating fresh water from seawater. Let’s break it down step by step:
1. Heating the seawater:
The process begins by placing seawater in a distillation flask and applying heat using a burner or heat source. The purpose of heating is to increase the temperature of the seawater and facilitate the vaporization of water molecules.
2. Vaporization:
As the seawater heats up, the water molecules gain energy and start to evaporate, forming water vapor. Other substances, such as salt, have higher boiling points and do not vaporize at the same temperature.
3. Condensation:
The water vapor generated from the seawater travels through a pipe or tube connected to the distillation flask. This pipe is surrounded by a condenser, which contains cold water circulating around it. The cold water in the condenser helps to lower the temperature of the water vapor, causing it to condense back into liquid form.
4. Collection of fresh water:
The condensed water vapor, now in the form of liquid, drips or flows down from the condenser into a receiving flask located on the right-hand side of the diagram. This collected liquid is fresh water, separated from the salt and impurities present in the seawater.
5. Separation of salt particles:
While the water vapor undergoes condensation, the remaining salt particles and impurities stay behind in the distillation flask. These substances have higher boiling points and do not vaporize, thus remaining trapped in the flask.
By utilizing the differences in boiling points between water and other substances, the distillation process allows for the separation and collection of fresh water, leaving behind the salt and impurities. This method is commonly used in desalination plants to produce fresh water from seawater or brackish water sources.
Fractional Distillation
Fractional distillation is a type of distillation process that involves separating a mixture into its individual components based on their boiling points by using a fractional column.
Uses: Refining fuel from crude oil.
How is fuel oil formed?
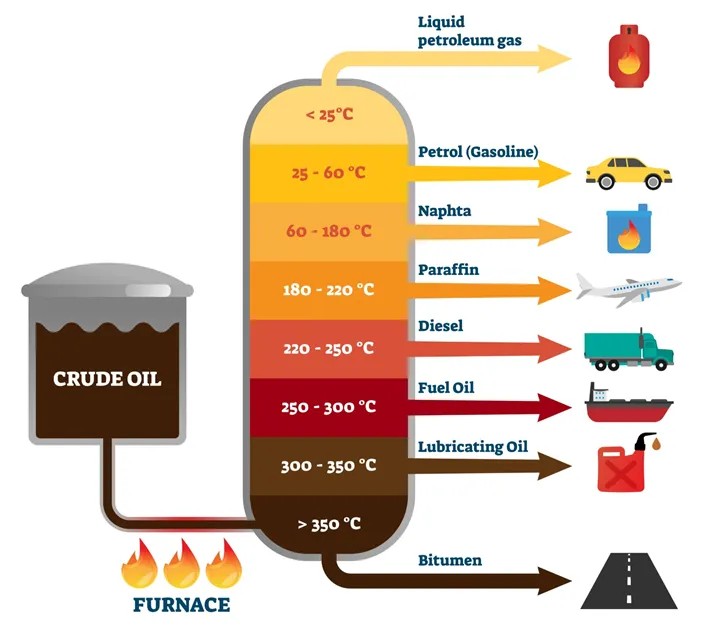
Fuel is formed from crude oil which is typically found in underground reservoirs, trapped within porous rocks such as sandstone or limestone. It is extracted through drilling wells into these reservoirs and then processed through refining to obtain various petroleum products such as gasoline, diesel, jet fuel, and other industrial chemicals.
When crude oil is needed to separate into further fuel oils it undergoes fractional distillation. From the above diagram you can see that crude oil is passed through the furnace and heated, all the fuel fractions of crude oil have different boiling points. When heated it starts vaporising and the vapour goes up then passes onto different levels of columns for different fractions of crude oil, where it is cooled down so that the vapour transforms into liquid state.
Fractions having lighter density like gases and petrol will pass from the first and second column respectively. The fractions having higher density will pass from the lower columns and at the bottom we are left with bitumen which will be used for building roads.
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/. It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.


[…] Naphthenes or cycloalkanes: Naphthenes or cycloalkanes are saturated hydrocarbons that have at least one ring of carbon […]