What is saturated solution? / What is unsaturated solution? / What is supersaturated solution?
You’re stirring a spoonful of sugar into your cup of tea. At first, it vanishes, dissolved into the liquid, leaving behind no trace of its crystal form. Have you ever wondered why there’s a limit to how much sugar your tea can take? Why, no matter how much you stir, there comes a point when it just won’t dissolve anymore? The answer lies in the solution.
Saturated solution:
Saturated-solutions is a solution in which no more solute can be dissolved at a certain temperature, any additional solute added will not dissolve and will settle at the bottom of the container. For dissolving more solute in this liquid, the liquid should be heated and then the solute can dissolve again in this solution.
Example: A classic example of a saturated solutions is sugar water. If you keep adding sugar to a glass of water and stirring until no more sugar can dissolve, you have a saturated sugar solutions. The excess sugar that doesn’t dissolve collects at the bottom of the glass.
Unsaturated solution:
Unsaturated solution is a solution where more solute can be added to the solution at a constant temperature. In other words, there is still room for more solute to dissolve in the solvent. Unsaturated solutions can be created by adding less solute than the solubility limit or by lowering the temperature, which reduces the solubility of the solute.
Example: Consider a glass of iced tea. If you dissolve a teaspoon of sugar in a glass of iced tea and it completely dissolves, you have an unsaturated sugar solution. There is still room for more sugar to dissolve in the tea.
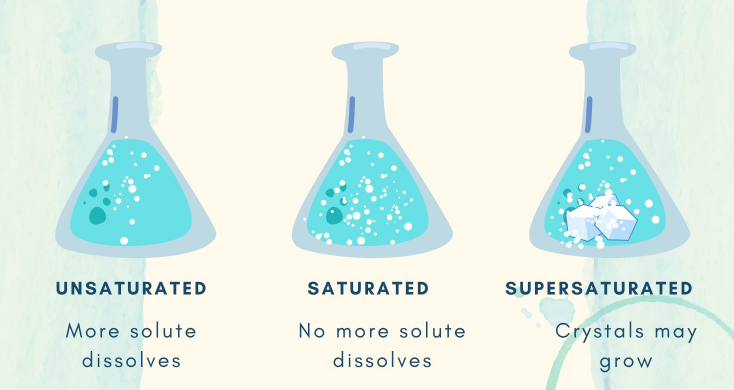
Supersaturated solution:
Supersaturated solution is a solution in which after the saturation point we dissolve more solute by increasing temperature. It is related to how much temperature increases and how much extra solute will dissolve with respect to temperature
For Example: At 30℃, 1 litre of water can dissolve 30 grams of sugar. And this same amount of water when heated up to 60℃ can dissolve 50 grams of sugar. This extra 20-gram sugar changes back into a semi-solid when the temperature again comes down to 30℃.
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://www.merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.