What are Gas laws? Types of Gas law | Application | Ideal gas law |Boyle’s law | Charle’s law | Gay lussac law |
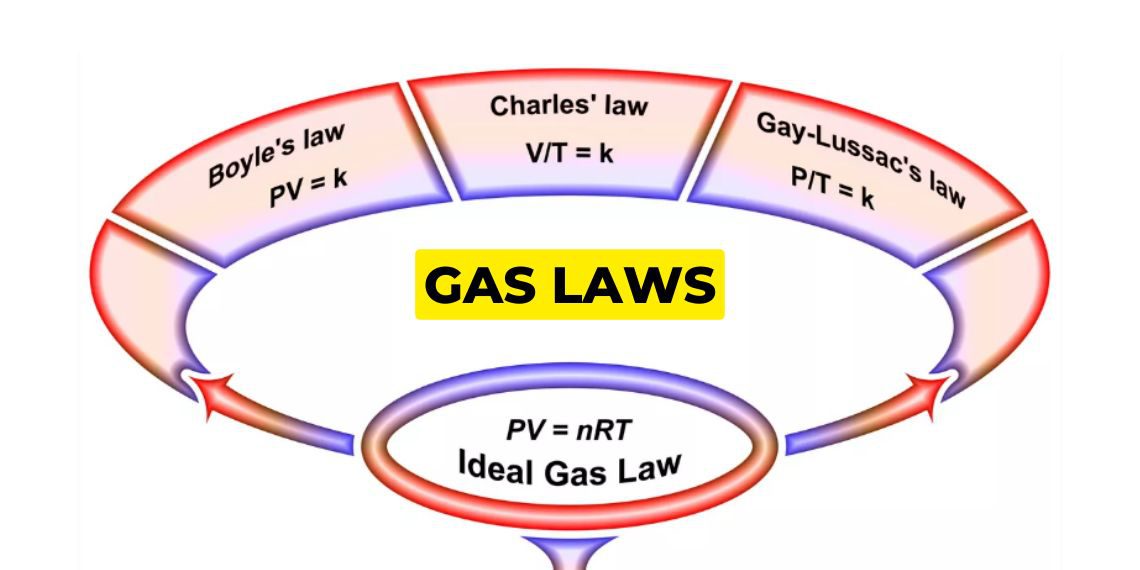
What are gas laws?
Gas laws are a group of laws that govern the behaviour of gases by providing relationships with volume, pressure, temperature, and no. of molecules.
Some of the important gas laws are:
Boyle’s law
Boyle’s law gives the relationship between a gas’s pressure and volume at a constant temperature. Basically, at constant temperature the volume of a gas is inversely proportional to the pressure of a gas at a constant temperature.
Example: Syringe- When we press the plunger the pressure increases due to which the volume decreases.


Charles law
Charles law states that at constant pressure, the volume of a gas is directly proportional to the temperature. (in kelvin) in a closed system. Basically, this law describes the relationship between the temperature and volume of the gas.
For example; a ballon in a warm room, if we take the ballon outside where the temperature is less then we will see that the ballon is shrinking. It’s because as the temperature reduces the volume of gas got reduced and when the ballon is brought back to the warm room we will see that the ballon is expanding.


Gay Lussac law
Gay Lussac law is a type of gas laws which gives the relationship between temperature and pressure at a constant volume. The law states that at a constant volume, the pressure of the gas is directly proportional to the temp, for a given gas.
For example, when heat is applied on a pressure cooker the volume remains constant. So the pressure will increase and after some time when the pressure becomes very high, the relief valve opens and releases the pressure. This shows that temperature is proportional to pressure.


Avogadro’s Law
Avogadro’s Law states that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. This law helps explain the concept of molar volume and the relationship between volume and the number of moles of gas.

Ideal gas law
The ideal gas law is the equation of the state of an ideal gas that relates pressure, volume, the quantity of gas, and absolute temperature.
PV=nRT
WHERE, P=pressure.
v=volume.
n=number of moles of gas.
R=ideal gas constant. (8.314 J/mol-k)
T=absolute temperature

| Gas Law | Formula | Description |
| Charles’s Law | V1/T1=V2/T2 | At constant P, as the volume increases the temperature also increases. |
| Boyle’s Law | P1V1=P2V2 | At constant T, if pressure increases then volume decreases. |
| Gay- Lussac Law | P1/T1=P2/T2 | At constant V as pressure increases the temperature also increases. |
| Avogadro’s Law | V / n = constant | When the amount of gas increases, the volume of the gas also increases at constant pressure and temperature |
| Ideal Gas Law | PV=nRT | Ideal gas is those which has no force of attraction and no force of repulsion (i.e., Intermolecular forces between particle will be zero ) |
Note:
If you want to learn more about this topic, we suggest checking out our Combo package with the given link https://merchantnavydecoded.com/courses/c/ . It’s a great way to dive deeper into the subject through video explanations. This package covers all the important details and presents them in an easy-to-understand format. Watching the videos will help you grasp the topic better and make learning more enjoyable. So, we highly recommend giving our Combo package a try to enhance your knowledge on the subject.
Disclaimer :- The opinions expressed in this article belong solely to the author and may not necessarily reflect those of Merchant Navy Decoded. We cannot guarantee the accuracy of the information provided and disclaim any responsibility for it. Data and visuals used are sourced from publicly available information and may not be authenticated by any regulatory body. Reviews and comments appearing on our blogs represent the opinions of individuals and do not necessarily reflect the views of Merchant Navy Decoded. We are not responsible for any loss or damage resulting from reliance on these reviews or comments.
Reproduction, copying, sharing, or use of the article or images in any form is strictly prohibited without prior permission from both the author and Merchant Navy Decoded.